
On October 19, 2021, FDA issued a proposal to establish a new category of OTC hearing aids. Its purpose is to make it easier for tens of millions of American hearing loss people to obtain hearing aid products and improve communication and life.
The proposal has just been released, and one stone has aroused thousands of waves. Many hearing aid factories, earphone brand manufacturers and chip manufacturers have begun to enter the market or lay out the OTC hearing aid market. Before that, the global hearing aid market was monopolized by five major manufacturers. Due to lack of competition, the price was expensive, and the technology was only developing slowly.
Not long ago, the FDA issued a proposal on the category of OTC hearing aids, which ushered in new opportunities for the hearing aid market. What new opportunities will earphone manufacturers have in the face of this change in the OTC hearing aid market?
1、Background of OTC hearing aid act
1. 1.5 billion people worldwide have hearing loss, and the wearing rate in China is less than 5%
According to US media sources, 37.5 million adults in the United States alone have hearing problems, while less than one in five people can use hearing aids. Looking at the world, this figure is even more shocking. According to the statistics of who (World Health Organization), 1.5 billion people worldwide suffer from hearing loss (Figure 1), and the wearing rate of hearing aids is much lower than that of the United States. For example, in China, the wearing rate of hearing aids among hearing impaired people in China is even lower than 5%.
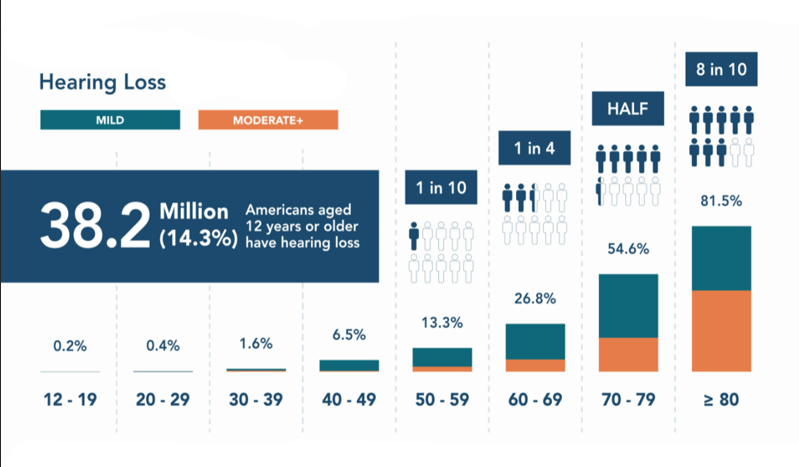
Figure 1: 1.5 billion people worldwide suffer from hearing loss
2. Significance of OTC hearing aid market opening
In the United States, buying traditional hearing aids requires a doctor's prescription. This standard places the sales and use of hearing aids under a relatively strict supervision to ensure the safety and effectiveness of users in the process of using hearing aids. But it also brings the closure of the market.
On the premise of ensuring the same safety and effectiveness of OTC hearing aids and traditional prescription hearing aids, the OTC bill will allow them to be sold directly to people with mild to moderate hearing loss through stores or online without the prescription of doctors or audiologists.
This can not only make it easier for hearing impaired people to buy hearing aid products, but also help increase market competition by reducing the threshold of sales channels and introducing more manufacturers.

3. Relationship between OTC hearing aid and hearing aid headset
The hearing aid is from the medical industry. The concept of auxiliary listening earphone is put forward more from the industry of consumer hearing products.
Hearing assistance devices, hearing devices, or hearing buses, in short, is an enhanced headset, which is suitable for people with mild hearing loss and some moderate hearing loss. It can realize some functions of hearing aids, such as segmented compression compensation of sound. It is mainly aimed at people with mild hearing loss caused by wearing headphones for a long time and listening to songs at a high volume, or people with gradual hearing loss due to age.
Figure
2: OTC and auxiliary earphones come from the medical industry and consumer industry respectively
From the perspective of users, the target customers of hearing aid headphones and OTC hearing aids are those with mild to moderate hearing loss (Figure 2). From the perspective of fate, OTC's proposal comes from the medical hearing aid industry, which should more strictly abide by the safety and effectiveness guarantee specifications, while the concept of hearing aid headphones comes more from the consumer electronics market and complies with the specifications of general electronic products. In terms of normative and technical requirements, medical grade products are higher than consumer electronic products. In terms of core technical indicators, hearing aid headphones follow the standard of OTC hearing aids.
2、Opportunities for domestic headphone manufacturers: new foreign markets and differentiated competition of TWS headphones
1. OTC hearing aids - incremental market opening
The new category of OTC hearing aids has opened a channel for the incremental market of hearing aids. Hearing loss users who were unable to buy hearing aids due to price, fitting, appearance and other reasons have greater opportunities to use hearing aids. At the same time, for new hearing aid manufacturers, there can be more channels to expand the market.
The direct impact on domestic hearing aids ODM and OEM manufacturers is the emergence of orders for OTC hearing aids in Europe and America, as well as the emergence of independent brands of new OTC hearing aids.
2. Differentiated competition of TWS -- hearing aid headset and health headset
With the competition of TWS earphones becoming more and more popular, light medical treatment and great health have become the development trend of earphones.
The most important function in this trend is to create differentiated high-end earphone products with hearing aid function and hearing health function as new selling points. With the introduction of OTC hearing aids, the market of hearing aid headphones and health headphones will be further expanded.
3、Standard interpretation of OTC hearing aids
1. Principle: meet the safety and effectiveness principles of medical devices, and give priority to meeting the safety needs.
Safety and effectiveness is the core principle for FDA to establish standards for medical devices. OTC hearing aids meet safety requirements by limiting the applicable population and input and output sound pressure. First, it is clearly stipulated that OTC hearing aids are only applicable to adults over the age of 18, so as to ensure that the risk of children under the age of 18 is minimized. The second is to limit the maximum output sound pressure. When the input is 90 dB SPL pure tone, the output limit of OTC hearing aid hearing aid at any sound frequency shall not exceed 115 dB SPL (with input compression and user adjustable control, it shall not exceed 120 dB SPL).

2. Specific technical indicators of OTC hearing aids
In the OTC standard proposal of FDA, the technical indicators are mainly reflected in two separate chapters. § 800.30 (d): output limits, and other requirements of § 800.30 (E) and (f).
(1) § 800.30 (d), output limits
FDA proposed the maximum sound output limit requirements of OTC hearing aids to provide reasonable guarantee for safety and effectiveness. High output may be unsafe and further damage hearing. However, too low output will reduce the effect of the device, and may lead to poor device performance, such as sound distortion, reducing consumer satisfaction. The proposed output limits in the proposal balance these considerations.
The output limit of OTC hearing aid is defined as the maximum sound output sound pressure level (SPL) of the device in the 2 - cm3 coupler. The corresponding measurement condition is that the device input is 90 dB SPL pure tone and the gain / volume switches are all on. The output of OTC hearing aids shall not exceed the following limits:
General output limits. The SPL output limit of OTC hearing aids at any sound frequency shall not exceed 115 dB SPL, except as specified in (2) below
OTC hearing aids with input controlled compression and user adjustable volume control shall not exceed the output limit of 120 dB SPL at any frequency.
(2) § 800.30 (E) and (f)
FDA believes that although output restrictions play a role in reasonably ensuring the safety and effectiveness of OTC hearing aids, these requirements alone are not enough. Therefore, FDA recommends that the equipment must meet certain performance and design requirements to help provide reasonable assurance of safety and effectiveness.
3. China hearing aids limits
4、OTC hearing aid solutions and solution providers
First, a professional R&D manufacturer. Specialized in manufacturing ITE, BTE hearing aids with high quality & good service.
ENNO provide as high quality and competitive price of hearing aids for who have hearing loss, and a hearing aid usually the best option to help correct hearing loss and a high quality of life. ENNO have a high qualification of R&D team, as the R&D Director is Japanese who was working in Sony Group’s Electronics. As the technicians with more than 30 years of experience for Sony R&D products, and the quality inspection to ensures ENNO hearing aids quality;
High quality customers very interested for the following ENNO hearing aids:

Copyright © 2025 Guangdong ENNO Medical Technology Co., Ltd., All Rights Reserved.